 |
 |
- Search
| Qual Improv Health Care > Volume 27(2); 2021 > Article |
|
Abstract
Purpose
Patients with hematologic malignancy (HM) typically have a high mortality rate when their condition deteriorates. The chronic progressive course of the disease makes it difficult to assess the effect of intervention on acute events. We investigated the effectiveness of a rapid response team (RRT) on in-hospital mortality in patients with HM.
Methods
We retrospectively analyzed the data of patients with HM who admitted to the medical intensive care unit between 2006 and 2015. Clinical outcomes before and after RRT implementation were evaluated.
Results
A total of 228 patients in the pre-RRT period and 781 patients in the post-RRT period were included. The overall in-hospital mortality was 55.4%. Patients in the post-RRT period had improved survival; however, they required more vasopressor therapy, continuous renal replacement therapy, and extracorporeal membrane oxygenation. Multivariate analysis revealed that in-hospital mortality was associated with RRT activation (hazard ratio [HR], 0.634; 95% confidence interval [CI], 0.498–0.807; p < .001), neurological disease (HR, 2.007; 95% CI, 1.439–2.800; p < .001), sequential organ failure assessment score (HR, 1.085; 95% CI, 1.057–1.112; p < .001), need for continuous renal replacement therapy (HR, 1.608; 95% CI, 1.206–1.895; p< .001), mechanical ventilation (HR, 1.512; 95% CI, 1.206–1.895; p< .001), vasopressor (HR, 1.598; 95% CI, 1.105–2.311; p = .013), and extracorporeal membrane oxygenation (HR, 1.728; 95% CI, 1.105–2.311; p = .030).
Recent advances in treatment have improved clinical outcomes in patients with hematologic malignancy (HM) [1-5]. However, patients with HM typically have a high mortality rate when their condition deteriorates, which leads to them requiring intensive care unit (ICU) admission [6-8]. As patients with HM often have a severe, refractory immunocompromised status, sepsis rapidly progresses to a fulminant course.
Rapid response teams (RRTs) are implemented to detect early signs of physiological change before clinical deterioration and to provide interventions that might alter the deterioration trajectory. Several studies have reported that RRTs reduce in-hospital mortality and cardiopulmonary arrest rates [9-11]. However, heterogeneous study populations, such as non-surgical or cancer patients, are an important factor that might reduce the effectiveness of RRTs on survival, as well as the activation quality of RRTs [12-14]. For patients with HM, illness severity and prolonged hospital stays make it difficult to prove the efficacy of early intervention.
To date, few studies have investigated the utilization and outcomes of RRTs in patients with HM. Therefore, we investigated the effectiveness of RRTs on in-hospital mortality in patients with HM who admitted to the ICU.
In-hospital mortality and other clinical outcomes, including ICU mortality, one-month, three-month, six-month, and one-year mortalities, and length of ICU stay, in the post-RRT period were compared to those in the pre-RRT period. The study was conducted at a tertiary referral teaching hospital in Seoul, South Korea. The hospital has 2,704 beds, including 28 medical ICU beds. We retrospectively collected the clinical medical records of adult patients with HM, including leukemia, lymphoma, aplastic anemia, and multiple myeloma, who admitted to the ICU between April 2006 and June 2015. Data cleansing of ICU data has been available since April 2006, and the date of data extraction was early 2016. Patients who died within 24 hours of ICU admission were excluded. We extracted medical records from the Asan Biomedical Research Environment, a de-identified clinical data warehouse. Multiple readmissions to the ICU of individual patients were considered as separate cases.
The hospital RRT, composed of doctors and nurses specializing in intensive care medicine, was implemented in March 2009. The clinical outcomes of patients in the pre-RRT period, from April 2006 to February 2009, and the post-RRT period, from March 2009 to June 2015, were compared. The RRT has provided 24 hour/day coverage in all departments, including the emergency room, except for pediatrics (Figure 1). Details of the RRT activation criteria are provided in the supplemental Table 1. The RRT is triggered by a 24-hour electronic medical record-based screening system or a call from bedside medical teams. The RRT provides early resuscitation for shock, respiratory care, including advanced airway management, and cardiopulmonary resuscitation. Once the RRT is activated, the team performs all management under the instruction of ICU staff until the patient is transferred to the ICU. All decisions regarding ICU transfer are made by the RRT. The RRT discusses the do-not-resuscitate order in the case of end-stage HM or futile admission. The study design was approved by the Institutional Review Board of Asan Medical Center, which waived the requirement for informed consent due to the de-identified and retrospective nature of the study (project identification number 2015-1015) [15,16].
Baseline demographics, including age, sex, and comorbidities, were collected from electronic medical records. Sequential organ failure assessment (SOFA) scores were calculated using the worst parameters measured during the first 24 hours. We created a program to extract data from each item of the SOFA. Levels of intensive care support were assessed, including if the patient was supported by mechanical ventilation, vasopressors, continuous renal replacement therapy (CRRT), or extracorporeal membrane oxygenation (ECMO) during their ICU stay. Comorbidities were investigated based on claims data using the Korean Classification of Diseases 6. Patients with claims using diagnosis code G00-G99 were regarded as having neurologic disease and those with I00-I99 were regarded as having cardiac disease. The date of death was assessed based on the expiry date of national health insurance coverage. Deaths occurring within 24 hours of hospital discharge were categorized as in-hospital deaths.
Either Pearson’s chi-square test or Fisher’s exact test was used to compare categorical variables and either Student’s t-test or the Mann-Whitney test was used to compare continuous variables. Survival curves were plotted using the Kaplan-Meier method and were compared using a log-rank test. Hazard ratios (HRs) for univariate and multivariate survival analyses were calculated using the Cox proportional hazard model. All tests of significance were two-sided and differences among groups were considered significant when the p-value was < .05. All statistical analyses were performed using the SPSS software (version 22.0; IBM Corp., Armonk, NY, USA).
A total of 1,009 patients, 228 patients in the preRRT period and 781 patients in the post-RRT period, were included in the present study. The baseline characteristics of the patients are shown in Table 1. The mean age of the patients was 54.8 ±15.4 years old; 614 (60.9%) were men. There was no difference in comorbidities, except for a significantly higher incidence of airway disease in the post-RRT period (2.2% vs. 7.3%, p = .005). There was no difference in the SOFA score on ICU admission (9.1±3.8 vs. 8.9±3.9, p = .623). Survival was assessed one year after ICU admission unless the patient died within one year.
The overall in-hospital mortality rate of the study population was 55.4% (559/1,009) (Table 2). There were significant differences in in-hospital, onemonth, three-month, six-month, and one-year mortalities between the two groups. However, there was no significant difference in ICU mortality between the two groups. During ICU stay, significantly more patients in the post-RRT period required vasopressor therapy (75.4% vs. 84.9%, p = .001), CRRT (26.3% vs. 34.1%, p = .028), and ECMO (0 vs. 3.2%, p = .006) compared with those in the preRRT period. There was no difference in the length of ICU stay between the two groups. In-hospital survivors required significantly less vasopressor therapy, mechanical ventilation, CRRT, and ECMO, and had a lower SOFA score on ICU admission compared with non-survivors (Table 3). In contrast, in-hospital survivors were associated with RRT activation (p = .007). In-hospital survivors in the post-RRT period required significantly more vasopressor therapy (p = .002) and CRRT (p = .005) than those in the pre-RRT period, without a significantly different initial SOFA score (7.5±3.5 vs. 7.7±3.0, p = .819).
A Kaplan-Meier curve showed significantly improved patient survival in the post-RRT period compared with the pre-RRT period during the one-year follow-up period, with a median survival of 88.0 days (95% confidence interval [CI], 58.9–117.1) vs. 43.0 days (95% CI, 34.1–51.9, p < .001, Figure 2). Rapid response team activation was independently associated with in-hospital mortality on multivariate analysis (hazard ratio [HR], 0.634; 95% CI, 0.498–0.807; p < .001). Other independent determinants for in-hospital mortality were neurological disease (HR, 2.007; 95% CI, 1.439–2.800; p < .001), SOFA score (HR, 1.085; 95% CI, 1.057–1.112; p < .001), need for CRRT (HR, 1.608; 95% CI, 1.206–1.895; p < .001), mechanical ventilation (HR, 1.512; 95% CI, 1.206–1.895; p < .001), vasopressor therapy (HR, 1.598; 95% CI, 1.105–2.311; p = .013), and ECMO (HR, 1.728; 95% CI, 1.105–2.311; p = .030, Table 4).
In the present study, patients with HM who admitted to the ICU after the implementation of RRTs had significantly improved survival compared to those admitted before RRT implementation. Rapid response team activation was an independent determinant of in-hospital survival in multivariate analysis.
Significant advances in chemotherapy intensification for HM have increased not only clinical outcomes, but also the demand for intensive care. Due to their severe, refractory immunocompromised status and intensive chemotherapy, infectious complications are the main cause of morbidity and mortality in patients with HM or those undergoing stem cell transplantation [17-19]. Rapid response teams have been widely adopted over the last 20 years, providing early detection and intervention services that have reduced in-hospital mortality and cardiopulmonary arrest rates [9-11]. However, previous studies have indicated that RRTs were not as effective in patients with non-cardiac medical illness or malignancy as they were in surgically ill patients [12,14]. In our study, improved survival after RRT implementation in patients with HM indicates that this population may benefit from early intervention, regardless of illness severity or the disease’s chronic course. We assumed that the benefits of RRT activation come from early intervention by specially trained intensivists in these high-risk patients because immunosuppressive patients experience deterioration and death in a dramatically rapid fashion [20-22]. In addition, the improvement of intensive care has enabled aggressive treatments, such as CRRT, mechanical ventilation, and ECMO. These various factors may be associated with improved in-hospital survival rates. In the present study, the SOFA score on ICU admission was significantly associated with in-hospital mortality. The SOFA score is a simple and objective score that evaluates the amount and severity of organ dysfunction [23]. A previous study reported that the initial and highest SOFA scores were associated with mortality, while other studies emphasized that a change in SOFA score on days three or five was an important prognostic factor, independent of the initial SOFA score, in patients with HM [24-26]. Although we did not perform a serial evaluation of the SOFA score, we assumed that a greater number of patients in the post-RRT period might have had an increase in SOFA score early in the ICU stay compared with the pre-RRT period patients, considering that significantly more vasopressors, as well as increased CRRT and ECMO, were applied during the post-RRT period.
Multivariate analysis showed that the need for invasive mechanical ventilation, CRRT, vasopressor therapy, and ECMO were important determinants of in-hospital survival. Respiratory failure is well known to be associated with mortality in patients with HM [24,25,27-29]. The HEMA-ICU Study Group demonstrated that respiratory failure was the strongest predictor of one-year survival in patients with hematologic disease. Patients with both respiratory failure and acute kidney injury demonstrated a 19% survival rate, whereas patients without respiratory failure had a 54% survival rate [29]. The present study showed similar outcomes, as patients who needed both mechanical ventilation and CRRT showed a high one-year mortality rate. We assumed that early intervention for respiratory distress might prevent not only severe respiratory failure, but also respiratory complications and sequelae, which might be associated with lower long-term mortality.
The present study had several limitations. First, this study lacked important hematologic predictive factors, including the levels of bone marrow suppression, the time of the last chemotherapy, and disease status [30,31]. As we extracted data from a de-identified clinical data warehouse, detailed clinical and laboratory findings were not available. The reason for ICU admission is needed for a more accurate comparison of disease severity between the two groups. Second, the retrospective, single-center study design of our study may have limited the generalizability of our findings, although a large number of patients were included. Third, there were no comparative data on whether RRT activation affected the clinical outcomes. The improved quality of intensive care might have led to improved survival because this study was a longitudinal study over 10 years. More aggressive treatments, such as CRRT, mechanical ventilation, and ECMO, were provided in the post-RRT period. Previous studies have reported improved survival of patients with HM over the last decade [32-34]. Due to the long-term trend of declining in-hospital mortality rates, these decreases could not be unambiguously attributed to RRT activation. Although RRT was a significant prognostic determinant in the multivariate analysis, further direct comparative research is needed to clarify its impact on survival in patients with HM. Fourth, we only included patients who admitted to the ICU. An RRT may restrict patients with end-stage HM from being admitted to the ICU, which may have reduced mortality in the post-RRT period. In addition, we did not evaluate the clinical outcomes of patients who were successfully resuscitated in the general ward before the occurrence of severe deterioration due to early intervention. Fifth, the RRT increased by one member of ICU staff in 2010 and one intensivist in 2012, and the RRT system has been stabilized under the instruction of ICU staff since 2012. These personnel changes might have affected the quality of the RRT during the study period. There was no change in the number of nurses and equipment in the RRT. We could not evaluate the qualitative and quantitative personnel changes in the hematology ward.
In conclusion, RRT activation might be associated with improved survival in patients with HM, although advances in intensive care might also have affected survival. Further research, with detailed information on the level of bone marrow suppression and the cause of septic shock, is needed to identify subgroups of hematologic cancer patients who benefit from RRT activation to maximize the effective use of limited resources.
Figure 1.
Diagram of the activation of rapid response team. Details of the RRT activation criteria are shown in supplementary table 1. CPR, cardiopulmonary resuscitation.
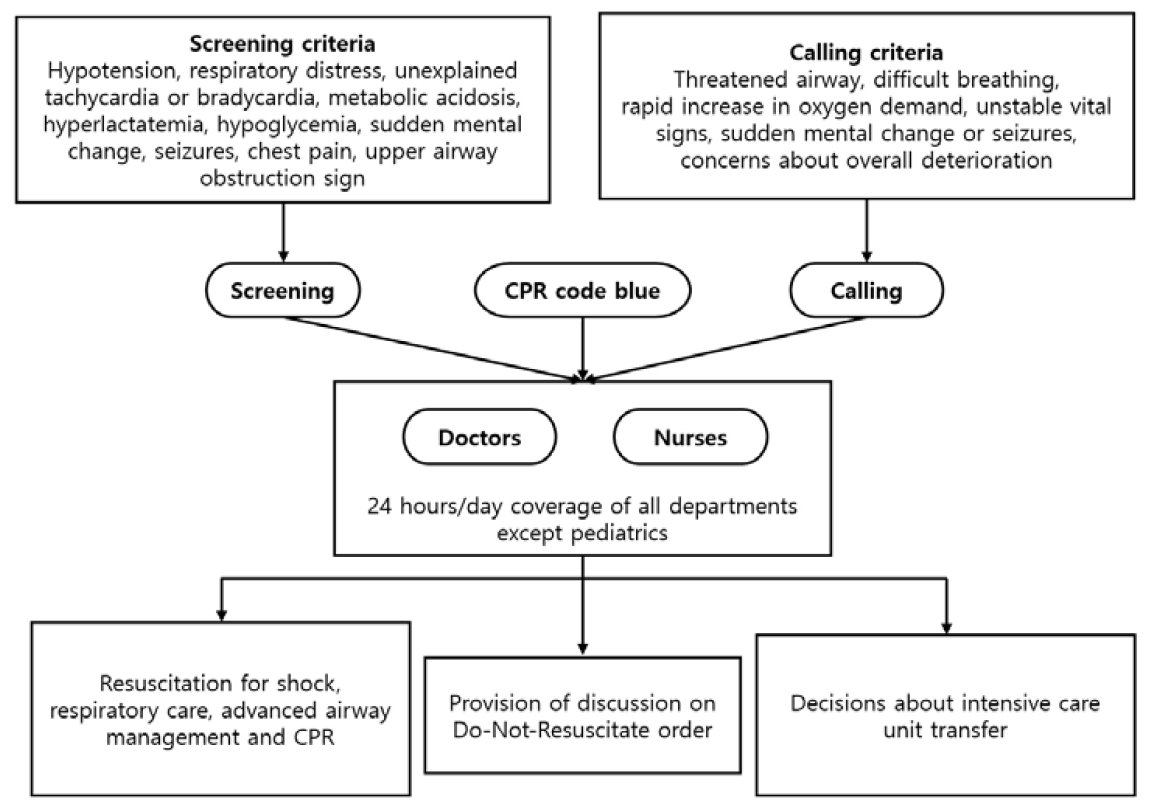
Figure 2.
Kaplan-Meier curve shows improved survival in the post-RRT period compared with the pre-RRT period during one-year follow-up period. RRT, rapid response team.
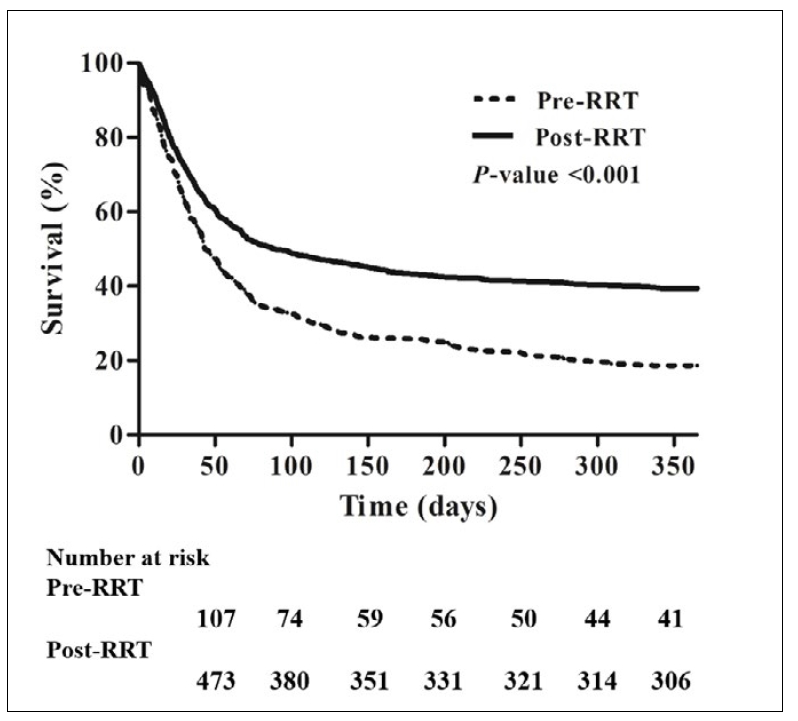
Table 1.
Baseline patient characteristics.
Table 2.
Clinical outcomes and levels of intensive care support before and after the rapid response team activation.
Table 3.
Comparison between in-hospital survivors and non-survivors in patients with hematologic malignancy.
Table 4.
Univariate and multivariate analysis for factors associated with in-hospital mortality.
Ⅴ. References
1. Pulte D, Gondos A, Brenner H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica. 2008;93(4):594-600. PMID: 18322250


2. Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113(7):1408-11. PMID: 18974371


3. Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111(10):4916-21. PMID: 18309034


4. Brenner H, Gondos A, Pulte D. Recent trends in long-term survival of patients with chronic myelocytic leukemia: disclosing the impact of advances in therapy on the population level. Haematologica. 2008;93(10):1544-53. PMID: 18641022


5. Al‐-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. NonHodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the national cancer data base from 1998 to 2011. American Journal of Hematology. 2015;90(9):790-5. PMID: 26096944


6. Oeyen SG, Benoit DD, Annemans L, Depuydt PO, Van Belle SJ, Troisi RI, et al. Long-term outcomes and quality of life in critically ill patients with hematological or solid malignancies: a single center study. Intensive Care Medicine. 2012;39(5):889-98. PMID: 23248039


7. Barreto LM, Torga JP, Coelho SV, Nobre V. Main characteristics observed in patients with hematologic diseases admitted to an intensive care unit of a Brazilian university hospital. Revista Brasileira de Terapia Intensiva. 2015;27(3):212-9. PMID: 26331970



8. Maqsood S, Badar F, Hameed A. Characteristics and outcomes of patients with hematological malignancies admitted for intensive care - a single centre experience. Asian Pacific Journal of Cancer Prevention. 2017;18(7):1833-7. PMID: 28749114


9. Jones D, Bellomo R, Bates S, Warrillow S, Goldsmith D, Hart G, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Critical Care. 2005;9(6):R808-15. PMID: 16356230


10. Chen J, Bellomo R, Flabouris A, Hillman K, Finfer S, MERIT Study Investigators for the Simpson Centre, ANZICS Clinical Trials Group. The relationship between early emergency team calls and serious adverse events. Critical Care Medicine. 2009;37(1):148-53. PMID: 19050625


11. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Critical Care. 2015;19(1):254. PMID: 26070457



12. Lee SH, Lim C-M, Koh Y, Hong S-B, Huh JW. Effect of an electronic medical record-based screening system on a rapid response system: 8-years’ experience of a single center cohort. Journal of Clinical Medicine. 2020;9(2):383. 

13. Lee J, Ban WH, Kim SW, Kim EY, Han MR, Kim SC. Utilization of a rapid response team and associated outcomes in patients with malignancy. Acute and Critical Care. 2020;35(1):16-23. PMID: 32131577



14. Shappell C, Snyder A, Edelson DP, Churpek MM, American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Predictors of in-hospital mortality after rapid response team calls in a 274 hospital nationwide sample. Critical Care Medicine. 2018;46(7):1041-8. PMID: 29293147



15. Shin S-Y, Lyu Y, Shin Y, Choi HJ, Park J, Kim W-S, et al. Lessons learned from development of de-identification system for biomedical research in a Korean tertiary hospital. Healthcare Informatics Research. 2013;19(2):102-9. PMID: 23882415



16. Choi HJ, Lee MJ, Choi C-M, Lee J, Shin S-Y, Lyu Y, et al. Establishing the role of honest broker: bridging the gap between protecting personal health data and clinical research efficiency. PeerJ. 2015;3:e1506. PMID: 26713253



17. Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. New England Journal of Medicine. 2010;363(22):2091-101. PMID: 21105791



18. Garcia JB, Lei X, Wierda W, Cortes JE, Dickey BF, Evans SE, et al. Pneumonia during remission induction chemotherapy in patients with acute leukemia. Annals of the American Thoracic Society. 2013;10(5):432-72. PMID: 23987587



19. Cannas G, Pautas C, Raffoux E, Quesnel B, de Botton S, de Revel T, et al. Infectious complications in adult acute myeloid leukemia: analysis of the acute leukemia French association-9802 prospective multicenter clinical trial. Leuk Lymphoma. 2012;53(6):1068-76. PMID: 22145959


20. Song JU, Suh GY, Park HY, Lim SY, Han SG, Kang YR, et al. Early intervention on the outcomes in critically ill cancer patients admitted to invensive care units. Intensive Care Medicine. 2012;38(9):1505-13. PMID: 22592633

21. Azoulay E, Pène F, Darmon M, Lengliné E, Benoit D, Soares M, et al. Managing critically ill hematology patients: time to think differently. Blood Reviews. 2015;29(6):359-67. PMID: 25998991


22. McCaughan D, Roman E, Smith AG, Garry A, Johnson M, Patmore R, et al. Determinants of hospital death in haematological cancers: findings from a qualitative study. BMJ Supportive & Palliative Care. 2018;8(1):78-86. PMID: 28663341


23. Amaral ACK-B, Andrade FM, Moreno R, Artigas A, Cantraine F, Vincent J-L. Use of the sequential organ failure assessment score as a severity score. Intensive Care Medicine. 2005;31(2):243-9. PMID: 15668764


24. Vandijck DM, Depuydt PO, Offner FC, Nollet J, Peleman RA, Steel E, et al. Impact of organ dysfunction on mortality in ICU patients with hematologic malignancies. Intensive Care Medicine. 2010;36(10):1744-50. PMID: 20480137


25. Geerse DA, Span LFR, Pinto-Sietsma S-J, van Mook WNKA. Prognosis of patients with haematological malignancies admitted to the intensive care unit: sequential organ failure assessment (SOFA) trend is a powerful predictor of mortality. European Journal of Internal Medicine. 2011;22(1):57-61. PMID: 21238895


26. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. The Journal of American Medical Association. 2001;286(14):1754-62. PMID: 11594901


27. Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium— a groupe de recherche respiratoire en réanimation onco-hématologique study. Journal of Clinical Oncology. 2013;31(22):2810-8. PMID: 23752112


28. Alp E, Tok T, Kaynar L, Cevahir F, Akbudak IH, Gündoğan K, et al. Outcomes for haematological cancer patients admitted to an intensive care unit in a university hospital. Australian Critical Care. 2018;31(6):363-8. PMID: 29429570


29. de Vries VA, Müller MCA, Arbous MS, Biemond BJ, Blijlevens NMA, Kusadasi N, et al. Long-term outcome of patients with a hematologic malignancy and multiple organ failure admitted at the intensive care. Critical Care Medicine. 2019;47(2):e120-8. PMID: 30335623



30. Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Critical Care Medicine. 2003;31(1):104-12. PMID: 12545002


31. Lamia B, Hellot M-F, Girault C, Tamion F, Dachraoui F, Lenain P, et al. Changes in severity and organ failure scores as prognostic factors in onco-hematological malignancy patients admitted to the ICU. Intensive Care Medicine. 2006;32(10):1560-8. PMID: 16896863


32. van Vliet M, Verburg IWM, van den Boogaard M, de Keizer NF, Peek N, Blijlevens NMA, et al. Trends in admission prevalence, illness severity and survival of haematological patients treated in Dutch intensive care units. Intensive Care Medicine. 2014;40(9):1275-84. PMID: 24972886


33. Ostermann M, Ferrando-Vivas P, Gore C, Power S, Harrison D. Characteristics and outcome of cancer patients admitted to the ICU in England, Wales, and Northern Ireland and national trends between 1997 and 2013. Critical Care Medicine. 2017;45(10):1668-76. PMID: 28682838


34. Sauer CM, Dong J, Celi LA, Ramazzotti D. Improved survival of cancer patients admitted to the intensive care unit between 2002 and 2011 at a US teaching hospital. Cancer Research and Treatment. 2019;51(3):973-81. PMID: 30309220



- TOOLS






