Regional Differences in Access to Clinical Trials for Cancer in Korea
Article information
Abstract
Purpose
The ability to access clinical trials for cancer treatment is important. This study investigated whether regional differences exist in oncologic clinical trial protocols conducted in South Korea.
Methods
Records of all approved oncologic clinical trials conducted in 2019 were downloaded from the Republic of Korea Ministry of Food and Drug Safety. The study covered Seoul, the capital area, other metropolitan cities, and provincial areas. Descriptive statistics summarized the distribution patterns of clinical trials by region.
Results
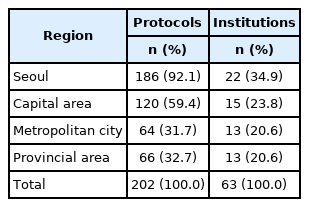
A total of 202 oncologic clinical trials were conducted in 63 institutions in 2019. Of these protocols, 186 (92%) were available in Seoul, 120 (59%) in the capital area, 64 (32%) in metropolitan cities, and 66 (33%) in provincial areas. More regional differences in protocol availability were observed in domestic trials, investigator-initiated trials, phase 1 and 2 trials, and smaller-scale trials.
Conclusion
Most oncologic clinical trials were conducted in medical institutions located in Seoul, with the rest conducted in the capital area, metropolitan cities, and provincial areas. The findings reveal clear differences in protocol availability between Seoul and the other regions. Measures designed to improve geographical access to oncologic clinical trials may be needed given their growing importance in cancer treatment.
Ⅰ. Introduction
Clinical trials are important for developing and implementing new oncologic drugs, particularly due to the constant development of new oncologic treatments, such as targeted therapy and immunotherapy. South Korea is known as one of the global hubs for clinical trials, a status accelerated by the Korea Good Clinical Practice legislation, the adoption of the International Council for Harmonization Good Clinical Practice standards, the introduction of a new Clinical Trial Authorization process, and the foundation of the non-profit Korea National Enterprise for Clinical Trials [1]. One of Korea’s particularly attractive characteristics as a global hub is its highly dense population concentrated in the capital area, in addition to the comparatively low costs and high availability of high-quality human resources [2]. Oncologic clinical trials are one of Korea’s strongest fields, with the highest proportion of drugs approved by the Ministry of Food and Drug Safety (MFDS) [3].
The development and implementation of oncologic clinical trials are promoted at the national level because they are essential for advancing cancer treatment and research. Due to their importance to novel cancer treatment, ensuring patient access to protocols is required in order to reduce potential cancer-related disparities [4]. However, reports show that many global industry-sponsored clinical trials tend to concentrate in large tertiary hospitals located in Seoul. This concentration may imply unequal access between patient populations according to their geographical area of residence or socioeconomic status, which may lead to disparities in patient outcomes [5]. Reducing disparities throughout the cancer continuum is an important cancer policy agenda; thus, the current geographical distribution patterns of oncologic clinical trials must be analyzed and monitored. This study addressed that need by analyzing the geographical distribution patterns of oncologic clinical trial protocols conducted in Korea to identify regional differences in protocol availability.
Ⅱ. Methods
This study used data downloaded from regular reports from the Republic of Korea’s MFDS, which lists all approved, ongoing, and completed clinical trials. A total of 974 protocols were registered in 2019, excluding bioequivalence studies and under-review protocols, of which 202 protocols were on cancer. The study identified and categorized all 202 approved, phase 1 to phase 3 protocols for cancer using automated methods and manual curation.
Korea is divided into 17 administrative regions. These were categorized into Seoul (capital city), the capital area (Incheon metropolitan city and Gyeonggi province), other metropolitan cities (Busan, Daegu, Daejeon, Gwangju, Sejong, and Ulsan), and provincial areas (Chungbuk, Chungnam, Gangwon, Gyeongbuk, Gyeongnam, Jeju, Jeonnam, and Jeonbuk). Descriptive statistics were used to summarize the study samples. Analysis was conducted using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Ⅲ. Results
Table 1 presents the general status of oncologic clinical trials conducted in 2019. A total of 202 clinical trials were conducted, of which 186 (92%) were conducted and available in Seoul, 120 (59%) in the capital area, 64 (32%) in metropolitan cities, and 66 (33%) in provincial areas. Trials were conducted in 63 institutions, including 22 institutions located in Seoul (35%), 15 (24%) in the capital area, 13 (21%) in metropolitan cities, and 13 (21%) in provincial areas.
Table 2 lists the characteristics of the analyzed protocols. The highest number of protocols were breast cancer (16.8%), followed by hematologic cancer (15.8%), and solid tumors (15.3%). More protocols were conducted by international (62.4%) than by purely domestic (37.6%) clinical trial institutions, and more were sponsor-initiated trials (SITs; 68.3%) than investigator-initiated trials (IITs; 31.2%). The highest number of protocols were phase 3 trials (33.2%), followed by phase 2 (30.7%) and phase 1 (12.9%) trials. Of the trials, 92 (45.5%) targeted 200 or more participants, 72 (35.6%) targeted fewer than 100 participants, and 38 (18.8%) targeted 100 to 199 participants. The tendencies found between regions (see Table 1) generally persisted regardless of cancer type, international status, sponsor type, phase, or the number of participants.
Ⅳ. Discussion
The results of this study show that most oncologic clinical trials are conducted in Seoul, followed by capital areas, metropolitan cities, and provincial areas. Differences in availability between Seoul and the other areas were noticeable, inferring potential disparities in the access to clinical trials. These differences generally persisted regardless of cancer type, sponsoring company, sponsor type, phase, or number of participants. Most of the protocols conducted outside Seoul and the capital area were largescale stage 3 international trials, aimed mainly at testing comparability, dosage, and safety. This implies that relatively novel research and protocols may tend to be centralized in Seoul.
One unique feature of the Korean healthcare system is that patients are mostly free to visit any medical institution of their choice [5]. Hence, patients tend to be concentrated in large, prestigious hospitals primarily located in Seoul. As these medical institutions provide extensive services and operate most of specialized clinical trial centers, vast differences exist in the availability of oncologic clinical trials between geographical regions [6]. Furthermore, as government support for clinical trials is limited, concentration is often preferred because it implies easier quality management, greater availability of human resources, and lower expenditures on contract research organizations [2]. Thus, the findings of this study may be a reflection of the current phenomenon in which cancer patients and oncological clinical trials tend to be concentrated in medical institutions located in the capital area.
Offering clinical trials as an alternative treatment is important, and the National Cancer Institute has stated that clinical trials are essential for evaluating the effectiveness of oncologic therapies [7]. Unsurprisingly, oncology constitutes the largest proportion of clinical trials for therapeutics in Korea, with international companies sponsoring multinational studies and local companies sponsoring domestic studies [3]. Structural factors, such as transportation costs, have been reported as a source of barriers to participation in clinical trials, while patient awareness and physician recommendation have shown correlations with a willingness to participate [4,8,9]. Under such circumstances, the geographical concentration of protocols in the capital area may imply geographical disparities in clinical trial access. In particular, individuals residing in rural areas or those belonging to vulnerable social groups may be affected by structural or financial barriers [10]. Considering the growing importance of clinical trials in cancer treatment, measures that improve geographical access to oncologic clinical trials may be needed.
Although these findings reveal geographical differences in protocol availability, they must be interpreted in the context of the following factors. First, the capital area covers more than 60% of cancer treatments, implying that more protocols are bound to be conducted there. Furthermore, phase 1 trials may be limited to the capital region because they require clinical pharmacology only a few medical institutions can offer. Likewise, the limited number of trials performed in provincial areas on certain cancer types, such as brain tumors, may be largely due to the unavailability of specialists. Therefore, further in-depth studies on these factors are needed.
Overall, this study found that most oncologic clinical trials are conducted in medical institutions located in Seoul, with the rest performed in the capital area, metropolitan cities, and provincial areas. These findings reveal clear differences in protocol availability between Seoul and the other regions. Measures for improving geographical access to oncologic clinical trials may be needed given their growing importance in cancer treatment.
Notes
Funding
None
Conflict of Interest
None